
EMA Marketing Authorization of New Drugs in January 2025
Shots:
-
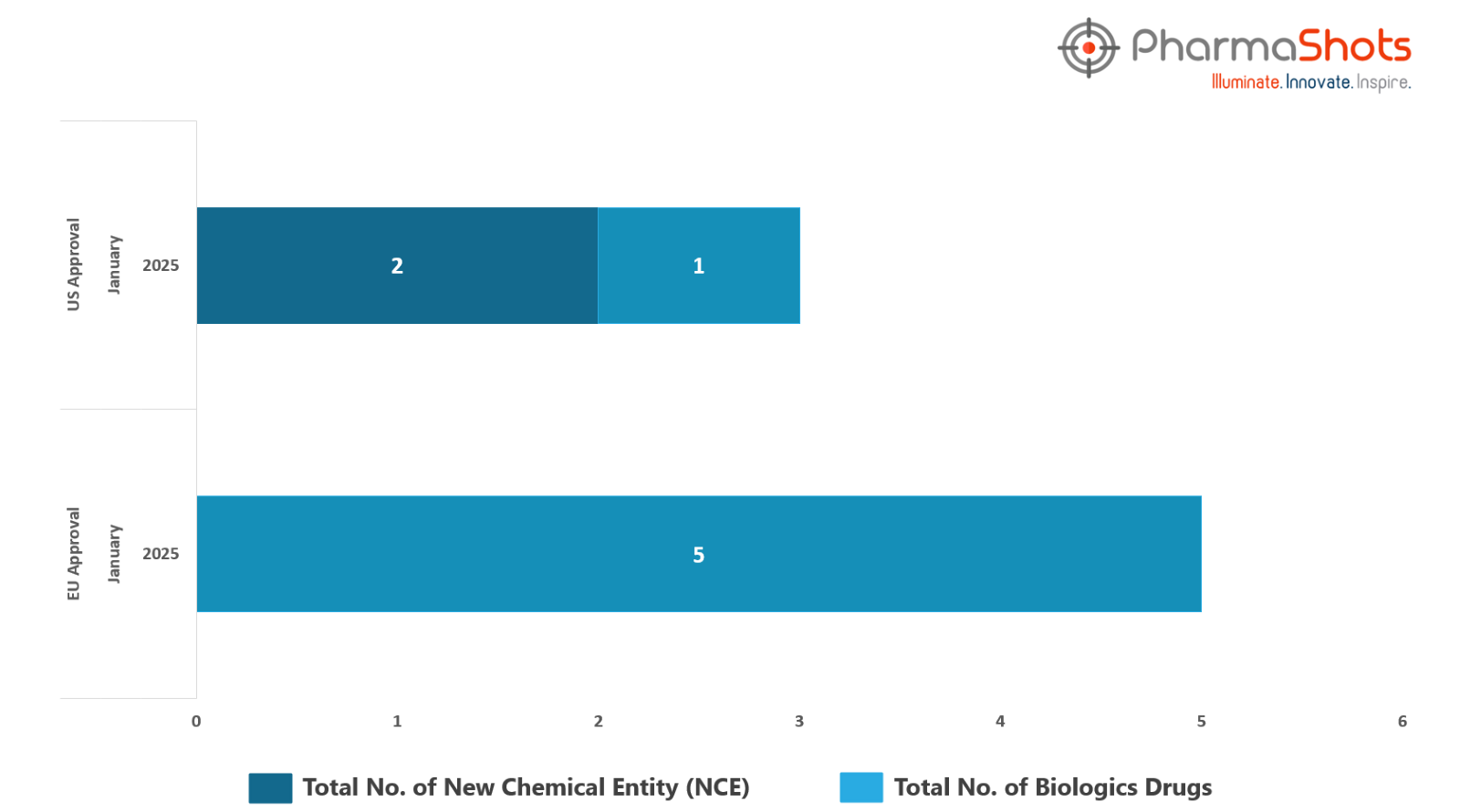
The EMA’s CHMP has granted positive opinions to 5 Biologics in January 2025, leading to treatments for patients and advances in the healthcare industry
-
The major highlighted drugs were Merck’s Capvaxive to treat Pneumococcal Disease
-
PharmaShots has compiled a list of 4 drugs that have been granted positive opinions and approvals by the EMA’s CHMP & EC, respectively
1. InflaRx’s Gohibic (Vilobelimab) Receives the EC Approval for the Treatment of ARDS
Company: InflaRx
Product: Gohibic
Active Ingredient: Vilobelimab
Disease: Acute Respiratory Distress Syndrome
Date: Jan 15, 2025
Shots:
-
The EC has approved (under exceptional circumstances) Gohibic to treat SARS-CoV-2-induced acute respiratory distress syndrome (ARDS) adults on SoC (incl. systemic corticosteroids) & IMV (with/without ECMO)
-
Approval was backed by results of the P-III (PANAMO) study assessing Gohibic vs. PBO among invasively mechanically ventilated COVID-19 patients in the ICU, which showed improvement in survival with a 23.9% relative reduction in 28-day all-cause mortality. The data was published in The Lancet Respiratory Medicine
-
The marketing authorization for GOHIBIC is valid in all 27 EU member states, Iceland, Liechtenstein, and Norway. InflaRx is exploring commercial partnerships in the EU
Company: Bavarian Nordic
Product: Vimkunya
Active Ingredient: CHIKV VLP vaccine
Disease: Chikungunya
Date: Jan 30, 2025
Shots:
-
The CHMP has recommended Vimkunya (pre-filled syringe) against CHIKV disease in individuals (≥12yrs.), with the EC’s decision & launch expected in H1’25 for the EU, Iceland, Liechtenstein, & Norway
-
Opinion was based on 2 P-III studies showing a rapid immune response in 1wk. with ~97.8% of participants (n=3,500) developing neutralizing antibodies by day 21
-
Following the positive CHMP opinion, the company will submit an MAA to the UK MHRA under the IRP, with potential UK approval in H1’25. Vaccine is also under priority review by the US FDA (PDUFA: Feb 14, 2025)
Company: Daiichi Sankyo and AstraZeneca
Product: Datroway
Active Ingredient: Datopotamab Deruxtecan-dlnk
Disease: Unresectable or Metastatic HR+/HER2- Breast Cancer
Date: Jan 30, 2025
Shots:
-
The CHMP has recommended Datroway to treat unresectable or metastatic HR+/HER2- (IHC 0, IHC 1+ or IHC 2+/ISH-) breast cancer in pts receiving endocrine-based therapy & CT. Ongoing regulatory review in China & other regions
-
Opinion was supported by a global P-III (TROPION Breast01) trial, assessing Datroway (6mg/kg; IV, Q21D) vs single agent CT (eribulin, capecitabine, vinorelbine or gemcitabine) in HR+/HER2- breast cancer pts (n=732)
-
Study showed improved PFS of 37%, mPFS (6.9 vs 4.9mos.), cORR (36% vs 23%) with CR (0.5% vs 0%) & PR (36% vs 23%), plus mDoR (6.7 vs 5.7mos). Data was published in JCO
Company: Merck
Product: Capvaxive
Active Ingredient: Pneumococcal 21-valent Conjugate Vaccine
Disease: Pneumococcal Disease
Date: Jan 30, 2025
Shots:
-
The CHMP has recommended Capvaxive for active immunization against invasive disease & pneumonia caused by Streptococcus pneumoniae in adults (≥18yrs.), with the EC’s decision expected in Q2’25 for EU, Iceland, Liechtenstein and Norway. Ongoing regulatory review in Japan, plus multiple filings are underway
-
Opinion was backed by P-III (STRIDE-3) data, assessing Capvaxive vs PCV20 in pneumococcal vaccine-naïve adults (≥18yrs.), plus data from various P-III trials (STRIDE-4, STRIDE-5, STRIDE-6, STRIDE-7, & STRIDE-10) assessing it in both vaccine-naïve & vaccinated adults
-
Capvaxive (single dose) targets Streptococcus pneumoniae serotypes against IPD in adults, incl. 8 unique serotypes (15A, 15C, 16F, 23A, 23B, 24F, 31, 35B)
Note: The following drugs have also been recommended for approval, however, no PR was available:
-
Tivdak (tisotumab vedotin)
Related Post: Insights+: EMA Marketing Authorization of New Drugs in December 2024
Tags

A passionate content writer with expertise in delivering high-quality and engaging content, Dipanshu is a keen reader and a versatile writer. Dipanshu dedicatedly covers news ranging from biopharma, life sciences, biotech, and MedTech to diagnostics and animal health companies, FDA, EMA, and biosimilar approvals. He can be contacted at connect@pharmashots.com














